| Issue |
Ann. For. Sci.
Volume 67, Number 8, December 2010
|
|
|---|---|---|
| Article Number | 809 | |
| Number of page(s) | 8 | |
| Section | Original articles | |
| DOI | https://doi.org/10.1051/forest/2010045 | |
| Published online | 28 October 2010 | |
Original article
Responses of wood anatomy and carbon isotope composition of Quercus pubescens saplings subjected to two consecutive years of summer drought
1
Institute of Plant Sciences and Oeschger Centre for Climate Change
Research (OCCR), University of Bern, Altenbergrain 21, 3013
Bern,
Switzerland
2
Swiss Federal Research Institute WSL, Zürcherstrasse 111, 8903
Birmensdorf,
Switzerland
3
Grup de Recerca en Biologia de les Plantes en Condicions
Mediterrànies, Departament de Biologia, Universitat de les Illes
Balears, Carretera de Valldemossa km
7.5, 07122
Palma de Mallorca,
Spain
4
Department of Geography, Johannes Gutenberg
University, Becherweg
21, 55099
Mainz,
Germany
* Corresponding author: alexander.galle@uib.es
Received:
1
December
2009
Accepted:
10
March
2010
• To withstand and to recover from severe summer drought is crucial for trees, as dry periods are predicted to occur more frequently over the coming decades.
• In order to better understand growth-related tree responses to drought, wood formation, vessel characteristics and stable carbon isotope composition (δ13C) in tree rings of Quercus pubescens saplings imposed to two consecutive summer droughts were compared with regularly watered control trees.
• In both years, photosynthetic activity was strongly inhibited during the drought periods of five to seven weeks but quickly restored after re-watering, reinitiating wood formation. Stress caused more than a 20% reduction in ring width, a 0.5‰ increase in latewood δ13C and changes in vessels characteristics in both the current year latewood and the next year earlywood. The latewood displayed up to 90% increased hydraulic conductivity than control trees, likely to compensate for a cavitation-induced reduction of water transport.
• The earlywood after the first drought year was characterized by more but smaller vessels suggesting the attempt of restoring conductivity while minimizing the risk of hydraulic failure. However, after the second year, the reduction of hydraulic conductivity and the increased δ13C values indicate a structural adjustment towards a reduced growth induced by exhaustion of carbon reserves.
Key words: carbon isotope composition / drought stress / earlywood and latewood / tree ring / wood anatomy
© INRA, EDP Sciences, 2010
1. Introduction
In the context of climate change extreme events like summer droughts and heatwaves are expected to occur more frequently in the near future, in particular across Europe (IPCC, 2007; Schär et al., 2004). As a result of the severity and duration of limited water availability, physiology, growth and survival of tree species will be adversely affected (Bréda et al., 2006; Ciais et al., 2005). Thus, the composition and survival of forests will depend on the variability of the climate, as well as on the response and adaptation strategies among the different tree species. Acclimation to drought consists of short- and long-term adjustments in physiology and morphology, which are often difficult to disentangle (Bréda et al., 2006; Kozlowski and Pallardy, 2002) and which also depend on the species.
During situations of drought, the shortage of water supply from the soil can cause hydraulic failure due to cavitation/embolism within xylem vessels (Hacke and Sperry, 2001; Sperry et al., 2008). One way to minimize the risk of xylem cavitation/embolism is by reducing water loss via the transpiration stream in leaves by closure of stomata, which is usually the first consequence of limited water supply and an internal adjustment to avoid rapid desiccation. In parallel, however, photosynthetic CO2 fixation is reduced. When these conditions persist (prolonged drought), tree carbon balance can become negative, as more carbon is needed (e.g. for respiration) than a tree can take up, resulting in decreased carbon availability and storage. Another way to cope with drought is to wait for favourable growing conditions and to repair hydraulic failures either by refilling cavitated vessels or by producing new vessels (Holbrook et al., 2001; Sperry et al., 2008). However, this process seems to require energy (carbon assimilates or stored carbohydrates) which might be limited after a long period of drought. Moreover, under prolonged drought the risk of irreversible damage by oxidative stress in photosynthesising leaves increases and thus tree species with a dynamic, adequate protection system might have an advantage (Chaves et al., 2003; Demmig-Adams and Adams, 2006). However, leaf-internal adjustments during times of impaired CO2 assimilation may become very costly, consuming previously fixed carbon for repair and protection processes. Although in the long run, slowed growth and reduction of leaf area are possible options to cope with limited water availability, especially for trees growing in dry habitats, shortage of carbon reserves seems to be the main cause of tree death after prolonged drought, as proposed by recent theories on tree mortality and forest dieback related to climate changes (Bréda et al., 2006; McDowell et al., 2008).
Growth responses to environmental changes are often registered and stored in the wood characteristics of the forming annual ring. For instance, stable carbon isotope composition (δ13C) of the wood (cellulose) can be used as a proxy for carbon storage and for water use efficiency (WUE), provided that photosynthetic CO2 fixation is directly transferred into the continuously developing tissue of tree rings (Leavitt and Long, 1986; O’Leary et al., 1992). It has been shown that δ13C is related to current and/or previous year photo-assimilated carbon (Ferrio et al., 2003; Kagawa et al., 2006; Livingston and Spittlehouse, 1996; Ponton et al., 2002; Skomarkova et al., 2006), which in turn reflects the hydric situation of the tree, thus serving as a surrogate for precipitation or soil water availability. Intra-annual variations of δ13C in early- and latewood may also be affected by internal factors like reallocation and storage processes (Helle and Schleser, 2004; Kagawa et al., 2006; Keel et al., 2007; Skomarkova et al., 2006). Similarly, wood anatomical characteristics can be used to understand the way in which the tree adjusts its hydraulic system to respond to a changing environment (e.g., Drew et al., 2009; Eilmann et al., 2006; Fonti et al., 2010; Gonzalez and Eckstein, 2003; Gruber et al., 2009; Lebourgeois et al., 2004). For example, during the dry period a reduction of lumen area and cell diameter has been observed for the tracheids produced in Abies balsamea (Rossi et al., 2009), as well as for the cross-sectional area of vessels elements in clones of Populus nigra (Arend and Fromm, 2007), Vitis vinifera (Lovisolo and Schubert, 1998) and in three Eucalyptus species (Searson et al., 2004). Similar reductions in oak vessel size (smaller and less vessels) have also been observed in the earlywood produced after a dry year (Eilmann et al., 2006; Lebourgeois et al., 2004; van der Werf et al., 2007).
These observations clarly indicate that both proxy (isotopes and cell anatomy) encode intra-annual information reflecting the physiological responses occurring during and after the drought stress.
In this study we intend to combine both approaches (isotopic and anatomy) with the aim of building a more complete picture explaining the responses occurring in trees enduring prolonged drought periods. To reduce the effect of other external factors influencing growth, we compared the differing responses in stable carbon isotope composition and vessel anatomical characteristics between potted pubescent oak trees (Quercus pubescens) which have been artificially subjected to two consecutive severe summer droughts and control potted trees growing outdoors.
2. Materials and methods
2.1. Plant material, experimental set up and climatic conditions
The experiment involved two groups of 6-year-old pubescent oak (Quercus pubescens Willd.) saplings, which were purchased from the cantonal nursery of Bern (origin of the Jura, Switzerland). The approximately 1.6 m high saplings were kept under natural condition in pots of 50 L on a flat field at the Botanical Garden of Bern, Switzerland from 2003 to 2006 (46° 57' 08' N, 7° 26' 42' E, 499 m a.s.l.). The soil was composed of 50% coarse clay, 40% brown soil and 10% humus. Plants were transferred to pots in 2003, fertilized and divided into two groups of 12 saplings each in a completely randomized design. During the summers of 2004 and 2005 the groups were subjected to two differing watering regimes. The “water-stressed group” (stress) was subjected to severe drought by withholding water until net photosynthesis was almost completely inhibited during the late morning and the early afternoon. The treatments were started when leaves had completely developed and matured. In total, water was withheld during 49 d in 2004 (from 2 July to 20 August) and 36 d in 2005 (13 June to 20 July) and re-watered to field capacity afterwards. The “control group” (control) was continuously watered to field capacity every two to three days during the experimental period, whereas during the rest of the year they were watered frequently (at least once a week). Changes in the pot weight of stressed and control plants were monitored weekly. Throughout the experiment many relevant physiological parameters – as predawn leaf water potential, leaf temperature, gas exchange, chlorophyll a fluorescence and various leaf compounds – were monitored on a weekly basis but more often during the late phase of drought and the initial phase of recovery, as presented in Galle et al. (2007). Since the present study focuses on drought-induced responses recorded in both wood anatomical characteristics and the 13C/12C isotope ratio (δ13C), wood samples from the stems of nine stressed and 10 control trees were collected in November 2006, i.e., one growing season after the experiment. Intact wood tissue was used for carbon isotope analysis since similar relationships between cellulose and bulk wood have been found (Harlow et al., 2006; Korol et al., 1999; Loader et al., 2003; Warren et al., 2001).
2.2. Measurement of wood anatomical characteristics
As wood anatomical characteristics, we considered the growth performance (ring widths) and the characteristics of the water conducting tissue (vessels). Measurements were thus performed on stem cross-sections of 10–12 μm thickness, which were taken from the stem base of each sapling and prepared with a sliding microtome. The cross-sections were stained with astrablue and safranin for better differentiation of cell types, and were fixed on microscope slides with Canada balsam for microscopic analyses. Pictures of cross-sections were taken with a CCD video camera mounted on a microscope with 40× optical magnification (Aristoplan, Leitz, Wetzlar, Germany) and measurements performed with the image analysis software ImageProPlus (Media Cybernetics, Silver Spring, USA). Measurements were performed along two 10 mm-wide and randomly selected radial bands. For each annual ring from 2003 to 2006, the ring width, number of vessels (NV), mean vessel lumen area (MVA) and total vessel lumen area (TVA) of earlywood (EW) and latewood (LW) were measured. In addition, the conductivity (CA) of EW and LW vessels was calculated according to Hagen-Poisseulle’s Law by taking vessel diameter to the fourth power (mm4) (Tyree and Zimmermann, 2002). Only vessels with a cross-sectional lumen area larger than 400 μm2 were considered for measurement, and all vessels with a lumen area larger than 5000 μm2 were assigned to EW vessels, according to Eilmann et al. (2006).
2.3. Measurement of stable carbon isotope ratios
Early- and latewood of each annual ring were separated under a binocular eyepiece according to the size of EW vessels with a scalpel from stem-wood sections of four plants per treatment group (control and stress) and ground to powder. Samples were combusted in an elemental analyser (Carlo-Erba, Rodano, Italy), CO2 was separated by chromatography and directly injected into a continuous-flow isotope ratio mass spectrometer (Thermo Delta Plus, Bremen, Germany). Peach leaf (NIST 1547) standards were run every six samples. The standard deviation of the analysis was below 0.1‰. The calculation of the 13C/12C isotope ratio (δ13C) was done as δ13Csample (‰) = (Rsample/Rstandard − 1) × 1000, where Rsample/Rstandard were referred to a Pee Dee Belemnite standard.
3. Results
3.1. Climatic conditions and severity of drought
The climatic conditions during the experimental period of 2004 and 2005 were typical of summer in the Swiss lowlands. The prevailing climatic conditions during the vegetative period (from April to October) in 2004, 2005 and 2006 consisted of similar minimum and maximum air temperatures and total rainfall, ranging between 0 °C and 32 °C and 680 mm, respectively (Tab. I). The mean air temperature during the drought treatments was 17.9 °C in 2004 (July to August) and 17.7 °C in 2005 (June to July). The total precipitation from May to September was 457 mm in 2004 and 499 mm in 2005.
Summary of some key climatic parameters recorded during the growing period of the summer green Q. pubescens from 2004 to 2006. T max and T min denote recorded maximum and minimum air temperatures, respectively. Monthly and total (SUM) rainfall is shown in mm from April to October.
During the imposed drought years, the loss of soil water was reflected in the progressive decline of pot weight over time. On a weekly basis the minimum pot weight and thus the maximum loss of extractable substrate water was reached in all treated plants after 6 and 4 weeks in 2004 and 2005, respectively. Thus, the tree to tree variation in reaching the minimum pot weight was within the range of six days (due to the time resolution of weighing), whereas variations in pot weight during drought treatments were generally small (less than 10%) among the trees. Almost no changes in pot weight were observed in controls trees throughout the whole experimental period. According to the depletion of soil water, predawn leaf water potential decreased gradually with ongoing stress and dropped to values below –3 MPa after between three and six weeks in 2004 and after two weeks in 2005. Changes in the predawn leaf water potential of the control plants were only small throughout the experimental period, ranging between –1.1 MPa and –0.2 MPa in both years. Besides an age-related increase of biomass in 2005, soil compaction and/or warmer climatic conditions after the start of the experiment in 2005 compared to 2004 might have contributed to an accelerated drought progression. After re-watering, soil and leaf water status were restored immediately in both years.
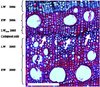 |
Figure 1 Representative cross-section with collapsed cell rows (indicated by white arrows) in the LW of stressed oaks. Differentiation in EW, LW and newly formed LW after severe drought stress (collapsed cells) is indicated on the lefthand scale. Red and blue coloured cell walls indicate dead (lignified) and living (non-lignified) cells, respectively. The white bar represents 1 mm. (A color version of this figure is available at www.afs-journal.org.) |
Clear signs of stress were observed in both the leaves and wood of drought-stressed trees, but no leaf losses occurred during and immediately after the stress. Leaf photosynthesis almost completely halted under prolonged water deficit, due to drought-induced closure of stomata and metabolic impairments (Galle et al., 2007). However, this photosynthetic inhibition was fully reversible after re-watering, due to the enhancement of photo-protective mechanisms. In wood, rows of collapsed cells were often observed within the LW (Fig. 1). When collapsed cells were observed, these were never found at the end of the ring, which indicates that wood formation resumed or continued after re-watering.
3.2. Drought effects on ring width and vessel characteristics
 |
Figure 2 Annual changes of ring width in well-watered (control; closed symbols) and drought-stressed (stress; open symbols) trees. Means and standard errors of control and drought-stressed trees are shown. Asterisks indicate significant differences between stress and corresponding control values (p ≤ 0.05). Insert depicts increment of radial stem growth since 2003. |
From 2003 to 2006, tree ring growth was highest in 2004 and decreased thereafter in 2005 and 2006, regardless of treatment group (Fig. 2). Drought-stressed trees, however, showed significantly smaller ring widths than control trees during the treatment years 2004 and 2005, as well as for the following year 2006 (p < 0.05, t-test). As compared to the controls, the ring widths of stressed trees were reduced by 22%, 30% and 38% in 2004, 2005 and 2006, respectively. With regard to the water-conducting tissue, we observed that the number of vessels (NV) within the EW of control trees increased progressively from 2003 to 2006 by more than twofold (Fig. 3a). A similar trend was found in drought-stressed trees until 2005, in which significantly more EW vessels were formed during drought years (2004 and 2005) than in controls. In 2006 however, a significant decline of NV of EW by more than 20% (t-test; p ≤ 0.05) was observed in stressed trees.
 |
Figure 3 Annual changes in wood characteristics in EW (circles) and LW (triangles) of control (closed symbols) and stressed oaks (open symbols) since 2003. NV, MVA, TVA and CA denote number of vessels, mean vessel area, total vessel area and conduit area, respectively. Means and standard errors of at least four trees per treatment are shown as a percentage of the starting situation (control) in 2003. Asterisks indicate significant differences between stress and corresponding control values (p ≤ 0.05). |
On the other hand, mean vessel area (MVA) of EW increased only slightly in stressed trees after 2003 (up to 120% in 2006), whereas the MVA of control trees increased to 170% in 2006 (Fig. 3c). In relation to the observed differences in the NV and MVA, the total vessel area (TVA) of EW increased similarly in control and stressed trees until 2005 (Fig. 3e). However, in 2006 the TVA differed significantly between stressed and control trees, due to a drop in stressed trees and an increase in control trees. As a consequence, the calculated conductivity (CA) of EW in control and stressed trees followed the same trend as for the TVA (Fig. 3g). The CA of all trees increased by two- and fivefold in 2004 and 2005, while in 2006 it increased only in the control trees (by tenfold since 2003).
As LW forms up to 90% of a tree ring in Q. pubescens, the NV of LW in stressed and control trees followed the trend of ring width, showing first an increase in 2004 and then a successive decrease in 2005 and 2006 (Fig. 3b). However, as opposed to the ring width data, from 2004 to 2006 the NV was always somewhat lower in the control than in stressed trees.
The MVA of LW decreased slightly in controls during 2004 and 2005 (followed by an increase in 2006), while the MVA in stressed trees remained almost unaltered (Fig. 3d). However, differences among stressed and control trees in the MVA were not significant.
The values of the TVA of LW followed the same trend as for the NV, with significantly higher values in stressed trees during 2004 (ca. 34%) and 2005 (ca. 90%) when compared to the controls (Fig. 3f). Similarly, the CA of LW remained always higher in stressed than in the comparable control trees, with significant higher values in 2004 and 2005 (Fig. 3h).
3.3. Drought effects on stable carbon isotope composition of wood
 |
Figure 4 Changes in carbon isotope composition (δ13C) in tree rings from 2003 to 2006. Means and standard errors of at least four trees per treatment are shown. Asterisks indicate significant differences between stress and corresponding control values (p ≤ 0.05). |
Drought-induced differences between the groups (control and drought-stressed trees) were observed for the isotopic signature of wood in tree rings, δ13C (Fig. 4). Whilst annual δ13C of the control trees remained very constant over the whole period at –26.04‰ ± 0.07, δ13C of drought-stressed trees from 2003 to 2006 showed less negative values compared to control trees, however significantly different only in 2004. Partitioning of δ13C in EW and LW revealed almost unaltered values for the control trees, while δ13C in the EW of stressed trees increased slightly during 2004 and 2005 (Fig. 5). Moreover, δ13C of LW in stressed plants increased from –26.2‰ (2003) to –25.5‰ (2006), differing significantly from the control trees in 2004. Overall, less negative values of annual δ13C in stressed trees were mainly impacted by LW, as δ13C values of EW were similar than in control trees.
 |
Figure 5 Changes in carbon isotope composition (δ13C) in EW and LW from 2003 to 2006. Means and standard errors of at least four trees per treatment are shown. Asterisks indicate significant differences between stress and corresponding control values (p ≤ 0.05). |
4. Discussion
Withholding water for about six to seven weeks during the summers of 2004 and 2005 resulted in distinct changes in tree physiological processes, as evidenced by changes in both wood characteristics and stable carbon isotope composition. Moreover, the observed rows of collapsed or damaged cells within the LW of either 2004 or 2005 annual rings indicate that the drought stress was severe enough to affect current growth processes by leaving a mark of the timing of drought occurrence during ring formation. The formation of additional latewood also indicates that stressed trees where able to resume growth once the re-watering started.
4.1. Immediate growth response to drought and re-watering
During the experiment as in the following year, plant growth was significantly reduced in comparison to the corresponding control plants, which suggests an immediate effect of water shortage on wood biomass production. This is in agreement with many studies on the tree-ring growth of deciduous and evergreen oaks subjected to drought (Corcuera et al., 2004; Eilmann et al., 2006; Tessier et al., 1994; Zweifel et al., 2006) and is directly related to the inhibition of photosynthetic activity under prolonged drought stress (Galle et al., 2007). However, although drought conditions were severe (predawn leaf water potential lower than –3 MPa), persistent (49 d in 2004 and 36 d in 2005) and late in the growing season (until 20 August in 2004 and 20 July in 2005), all trees were able to withstand the harsh conditions and resumed growth by building new layers of latewood cells. This strength is likely due to the ability of the species to preserve its photosynthetic apparatus during severe drought conditions (Haldimann et al., 2008), so that photosynthetic activity and hence the rate of CO2 assimilation are quickly restored after re-watering (Galle et al., 2007). The more positive δ13C values of current-year LW of stressed compared to control trees could be an indication of the limited photosynthetic CO2 assimilation caused by drought conditions, and/or due to the re-mobilization of previously stored carbon (Helle and Schleser, 2004; Kagawa et al., 2006; Skomarkova et al., 2006), and/or due to a higher WUE (about 10–20%, calculated from data of photosynthesis and stomatal conductance in Galle et al. 2007) during the onset of drought and after re-watering, which is in agreement with the proposed general positive relationship between WUE and δ13C (Farquhar et al., 1989). The slightly higher δ13C values observed in the non-stressed year 2006 might be related to both reduced growth (leaf biomass) and the leaf intrinsic adjustments (i.e. increased WUE) of stressed trees.
Drought or increasing soil water deficit usually causes cavitations within the xylem, i.e. the formation of air emboli in conduits that interrupt the upwards movement of water, which impairs water transport (Sperry et al., 2008; Tognetti et al., 1998; Tyree and Sperry, 1989). The newly formed LW is characterized by increased water conducting area and conductivity (TVA, CA), which increases the TVA of LW by about 10–20%. These facts suggest a compensating response which aims at increasing the efficiency of water transport from the roots via the stem to the photosynthesising leaves.
4.2. Long-term adjustment to drought
The growth reduction observed in the 2006 – i.e., the year following the experiment – is probably the result of lower carbon reserves and reduced hydraulic conductivity, although other impacts like root constraint and nutrient limitations cannot be ruled out completely.
Despite the reduced wood formation and the impaired carbon storage, the water conducting area and conductivity (TVA, CA) within the following-year EW was not affected after the first summer drought. However, more and smaller EW vessels (NV) were formed in stressed as compared to well-watered trees. This association might be considered as the first adjustment to maintain the efficiency of water transport, by minimizing the risk of xylem cavitations through an increased partitioning of EW into smaller vessels (Sperry et al., 2008; Tyree and Zimmermann, 2002). As shown in several studies (including Quercus species), a reduction of vessel size can be considered one way to lower the risk of xylem cavitations, especially under drought conditions (Cavender-Bares and Holbrook, 2001; Corcuera et al., 2006; Logullo et al., 1995).
However, no such effect on EW vessels was observed after two consecutive summer droughts, presumably as a result of carbon reserves exhaustion and/or other plant-internal adjustments requiring less water transport, e.g. the reduced number of branches, leaves and roots (Bréda et al., 2006; Kozlowski and Pallardy, 2002; Rood et al., 2000; Thomas and Gausling, 2000). Similar rates of photosynthesis and of stomatal conductance for water vapour under watered conditions in 2005 as compared to 2004 also support the idea that Q. pubescens was able to adjust its water supply for maintained productivity (carbon assimilation) and growth (Galle et al., 2007). Moreover, the increased vessel area within the LW of stressed trees might also be considered as an adjustment of the hydraulic system towards improved water supply to prevent possible drought impairments. However, harsher climatic conditions in 2005 and presumably reduced foliage led to a more rapid decline of photosynthetic activity and hence reduced carbon storage, although photosynthesis recovered completely after re-watering. Thus, despite the capacity of Q. pubescens to restore photosynthetic activity after relief of drought stress in two consecutive years, impaired photosynthetic carbon assimilation, enhanced protection and repair processes resulted in reduced growth (i.e. leaf biomass) and reduced wood formation with fewer vessels and more positive δ13C. Consequently, tree growth was slowed after two years of summer drought to ensure its survival and maintain growth at a lower rate.
5. Conclusions
Rebuilding water conducting elements (in LW) to restore or improve water uptake after severe summer drought in both years (2004 and 2005) has been found to be a direct (intra-annual) and compensating response to drought-induced damages (cavitation, embolism, collapsed cells), which most likely facilitated the restoration of leaf photosynthesis, apart from short-term adjustments at the leaf level (Galle et al., 2007). To compensate for the reduced growth (including carbon storage) and to improve future productivity as well as to minimize the risk of cavitation/embolism, more (but smaller) EW vessels were formed in the following year (Sperry et al., 2008). After another year of drought with reduced growth and impaired carbon balance, plants had to adjust or rather reduce their growth (less foliage – less carbon acquisition) in 2006, where large and numerous conducting vessels were no longer needed. These adjustments represent an adaptation to survive and to minimize the risk of future impairments by water deficit (Maseda and Fernandez, 2006; McDowell et al., 2008). Thus, Q. pubescens seems to be capable of withstanding and surviving two consecutive summer droughts by highly dynamic regulation of physiological and morphological traits (i.e. leaf photosynthesis and water conducting elements). However, due to the expected increase in extreme summer drought events during the next decades, it remains unclear whether Q. pubescens is capable to survive more frequent drought periods.
Acknowledgments
All members of Jan Espers group (University of Mainz, formerly Swiss Federal Research Institute WSL) are kindly acknowledged for their technical assistance and support. We are grateful to R. Alder and C. Ball from the Institute of Plant Sciences of the University of Bern, as well as to the gardeners of the Botanical Garden of Bern for looking after the oak trees. We also would like to thank the technical service of the Universitat de les Illes Balears in Palma (Spain) for providing the isotope ratio mass spectrometer. This work was partially supported by the “NCCR Climate” framework, a programme of the Swiss National Science Foundation.
References
- Arend M. and Fromm J., 2007. Seasonal change in the drought response of wood cell development in poplar. Tree Physiol. 27: 985–992. [PubMed] [Google Scholar]
- Bréda N., Huc R., Granier A., and Dreyer E., 2006. Temperate forest trees and stands under severe drought: a review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 63: 625–644. [CrossRef] [EDP Sciences] [Google Scholar]
- Cavender-Bares J. and Holbrook N.M., 2001. Hydraulic properties and freezing-induced cavitation in sympatric evergreen and deciduous oaks with, contrasting habitats. Plant Cell Environ. 24: 1243–1256. [Google Scholar]
- Chaves M.M., Maroco J.P., and Pereira J.S., 2003. Understanding plant responses to drought – From genes to the whole plant. Funct. Plant Biol. 30: 239–264. [CrossRef] [PubMed] [Google Scholar]
- Ciais P., Reichstein M., Viovy N., Granier A., Ogee J., Allard V., Aubinet M., Buchmann N., Bernhofer C., Carrara A., Chevallier F., De Noblet N., Friend A.D.,Friedlingstein P., Grunwald T., Heinesch B., Keronen P., Knohl A., Krinner G., Loustau D., Manca G., Matteucci G., Miglietta F., Ourcival J.M., Papale D., Pilegaard K., Rambal S., Seufert G., Soussana J.F., Sanz M.J., Schulze E.D., Vesala T., Valentini R., 2005. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 437: 529–533. [CrossRef] [PubMed] [Google Scholar]
- Corcuera L., Camarero J.J., and Gil-Pelegrin E., 2004. Effects of a severe drought on Quercus ilex radial growth and xylem anatomy. Trees 18: 83–92. [Google Scholar]
- Corcuera L., Camarero J.J., Siso S., and Gil-Pelegrin E., 2006. Radial-growth and wood-anatomical changes in overaged Quercus pyrenaica coppice stands: functional responses in a new Mediterranean landscape. Trees 20: 91–98. [CrossRef] [Google Scholar]
- Demmig-Adams B. and Adams W.W., 2006. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 172: 11–21. [CrossRef] [PubMed] [Google Scholar]
- Drew D.M., Downes G.M., O’Grady A.P., Read J., and Worledge D., 2009. High resolution temporal variation in wood properties in irrigated and non-irrigated Eucalyptus globulus. Ann. For. Sci. 66: 406. [CrossRef] [EDP Sciences] [Google Scholar]
- Eilmann B., Weber P., Rigling A., and Eckstein D., 2006. Growth reactions of Pinus sylvestris L. and Quercus pubescens Willd. to drought years at a xeric site in Valais, Switzerland. Dendrochronologia 23: 121–132. [CrossRef] [Google Scholar]
- Farquhar G.D.,Ehleringer J.R., and Hubick K.T., 1989. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40: 503–537. [Google Scholar]
- Ferrio J.P., Florit A., Vega A., Serrano L., and Voltas J., 2003. Delta 13C and tree-ring width reflect different drought responses in Quercus ilex and Pinus halepensis. Oecologia 137: 512–518. [CrossRef] [PubMed] [Google Scholar]
- Fonti P., von Arx G.,Garcia-Gonzalez I., Eilmann B.,Sass-Klaassen U., Gartner H., and Eckstein D., 2010. Studying global change through investigation of the plastic responses of xylem anatomy in tree rings. New Phytol. 185: 42–53. [CrossRef] [PubMed] [Google Scholar]
- Galle A., Haldimann P., and Feller U., 2007. Photosynthetic performance and water relations in young pubescent oak (Quercus pubescens) trees during drought stress and recovery. New Phytol. 174: 799–810. [CrossRef] [PubMed] [Google Scholar]
- Gonzalez I.G. and Eckstein D., 2003. Climatic signal of earlywood vessels of oak on a maritime site. Tree Physiol 23: 497–504. [PubMed] [Google Scholar]
- Gruber A., Zimmermann J., Wieser G., and Oberhuber W., 2009. Effects of climate variables on intra-annual stem radial increment in Pinus cembra (L.) along the alpine treeline ecotone. Ann. For. Sci. 66: 503. [CrossRef] [EDP Sciences] [PubMed] [Google Scholar]
- Hacke U.G. and Sperry J.S., 2001. Functional and ecological xylem anatomy. Perspect. Plant Ecol. Evol. Syst. 4: 97–115. [CrossRef] [Google Scholar]
- Haldimann P., Galle A., and Feller U., 2008. Impact of an exceptionally hot dry summer on photosynthetic traits in oak (Quercus pubescens) leaves. Tree Physiol. 28: 785–795. [PubMed] [Google Scholar]
- Harlow B.A., Marshall J.D., and Robinson A.P., 2006. A multi-species comparison of δ13C from whole wood, extractive-free wood and holocellulose. Tree Physiol. 26: 767–774. [PubMed] [Google Scholar]
- Helle G. and Schleser G.H., 2004. Beyond CO2-fixation by Rubisco – an interpretation of 13C/12C variations in tree rings from novel intra-seasonal studies on broad-leaf trees. Plant Cell Environ. 27: 367–380. [Google Scholar]
- Holbrook N.M., Ahrens E.T., Burns M.J., and Zwieniecki M.A., 2001. In vivo observation of cavitation and embolism repair using magnetic resonance imaging. Plant Physiol. 126: 27–31. [CrossRef] [PubMed] [Google Scholar]
- IPCC, 2007. Technical Report, Cambridge University Press, Cambridge and New York. [Google Scholar]
- Kagawa A., Sugimoto A., and Maximov T.C., 2006. 13CO2 pulse-labelling of photoassimilates reveals carbon allocation within and between tree rings. Plant Cell Environ. 29: 1571–1584. [CrossRef] [PubMed] [Google Scholar]
- Keel S.G., Siegwolf R.T.W., Jaggi M., and Korner C., 2007. Rapid mixing between old and new C pools in the canopy of mature forest trees. Plant Cell Environ 30: 963–972. [CrossRef] [PubMed] [Google Scholar]
- Korol R.L.,Kirschbaum M.U.F.,Farquhar G.D., and Jeffreys M., 1999. Effects of water status and soil fertility on the C-isotope signature in Pinus radiata. Tree Physiol. 19: 551–562. [PubMed] [Google Scholar]
- Kozlowski T.T. and Pallardy S.G., 2002. Acclimation and adaptive responses of woody plants to environmental stresses. Bot. Rev. 68: 270–334. [CrossRef] [Google Scholar]
- Leavitt S.W. and Long A., 1986. Stable-carbon isotope variability in tree foliage and wood. Ecology 67: 1002–1010. [CrossRef] [Google Scholar]
- Lebourgeois F., Cousseau G., and Ducos Y., 2004. Climate-tree-growth relationships of Quercus petraea Mill. stand in the Forest of Berce (“Futaie des Clos”, Sarthe, France). Ann For Sci 61: 361–372. [CrossRef] [EDP Sciences] [Google Scholar]
- Livingston N.J. and Spittlehouse D.L., 1996. Carbon isotope fractionation in tree ring early and late wood in relation to intra-growing season water balance. Plant Cell Environ. 19: 768–774. [Google Scholar]
- Loader N.J., Robertson I., and McCarroll D., 2003. Comparison of stable carbon isotope ratios in the whole wood, cellulose and lignin of oak tree-rings. Palaeogeogr. Palaeoclimatol. Palaeoecol. 196: 395–407. [CrossRef] [Google Scholar]
- Logullo M.A., Salleo S., Piaceri E.C., and Rosso R., 1995. Relations between Vulnerability to Xylem Embolism and Xylem Conduit Dimensions in Young Trees of Quercus cerris. Plant Cell Environ. 18: 661–669. [Google Scholar]
- Lovisolo C. and Schubert A., 1998. Effects of water stress on vessel size and xylem hydraulic conductivity in Vitis vinifera L. J. Exp. Bot. 49: 693–700. [CrossRef] [Google Scholar]
- Maseda P.H. and Fernandez R.J., 2006. Stay wet or else: three ways in which plants can adjust hydraulically to their environment. J. Exp. Bot. 57: 3963–3977. [CrossRef] [PubMed] [Google Scholar]
- McDowell N., Pockman W.T., Allen C.D., Breshears D.D., Cobb N., Kolb T., Plaut J., Sperry J., West A., Williams D.G., and Yepez E.A., 2008. Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought ? New Phytol. 178: 719–739. [CrossRef] [PubMed] [Google Scholar]
- O’Leary M.H., Madhavan S., and Paneth P., 1992. Physical and chemical basis of carbon isotope fractionation in plants. Plant Cell Environ. 15: 1099–1104. [Google Scholar]
- Ponton S., Dupouey J.L., Bréda N., and Dreyer E., 2002. Comparison of water-use efficiency of seedlings from two sympatric oak species: Genotype × environment interactions. Tree Physiol. 22: 413–422. [PubMed] [Google Scholar]
- Rood S.B., Patino S., Coombs K., and Tyree M.T., 2000. Branch sacrifice: cavitation-associated drought adaptation of riparian cottonwoods. Trees 14: 248–257. [Google Scholar]
- Rossi S., Simard S., Rathgeber C.B.K.,Deslauriers A., and De Zan C., 2009. Effects of a 20-day-long dry period on cambial and apical meristem growth in Abies balsamea seedlings. Trees 23: 85–93. [CrossRef] [Google Scholar]
- Schär C., Vidale P.L., Lüthi D., Frei C., Häberli C., Liniger M.A., and Appenzeller C., 2004. The role of increasing temperature variability in European summer heatwaves. Nature 427: 332–336. [CrossRef] [PubMed] [Google Scholar]
- Searson M.J., Thomas D.S., Montagu K.D., and Conroy J.P., 2004. Wood density and anatomy of water-limited eucalypts. Tree Physiol. 24: 1295–1302. [PubMed] [Google Scholar]
- Skomarkova M.V., Vaganov E.A., Mund M., Knohl A., Linke P., Boerner A., and Schulze E.D., 2006. Inter-annual and seasonal variability of radial growth, wood density and carbon isotope ratios in tree rings of beech (Fagus sylvatica) growing in Germany and Italy. Trees 20: 571–586. [CrossRef] [Google Scholar]
- Sperry J.S., Meinzer F.C., and McCulloh K.A., 2008. Safety and efficiency conflicts in hydraulic architecture: scaling from tissues to trees. Plant Cell Environ. 31: 632–645. [CrossRef] [PubMed] [Google Scholar]
- Tessier L., Nola P., and Serre-Bachet F., 1994. Deciduous Quercus in the Mediterranean region: tree-ring/climate relationships. New Phytol. 126: 355–367. [CrossRef] [Google Scholar]
- Thomas F.M. and Gausling T., 2000. Morphological and physiological responses of oak seedlings (Quercus petraea and Q. robur) to moderate drought. Ann. For. Sci. 57: 325–333. [CrossRef] [EDP Sciences] [Google Scholar]
- Tognetti R., Longobucco A., and Raschi A., 1998. Vulnerability of xylem to embolism in relation to plant hydraulic resistance in Quercus pubescens and Quercus ilex co-occurring in a Mediterranean coppice stand in central Italy. New Phytol. 139: 437–447. [CrossRef] [Google Scholar]
- Tyree M.T. and Sperry J.S., 1989. Vulnerability of xylem to cavitation and embolism. Annu. Rev. Plant Physiol. Plant Mol. Biol 40: 19–36. [Google Scholar]
- Tyree M.T. and Zimmermann M.H., 2002. Xylem structure and the ascent of sap. Springer, Berlin. [Google Scholar]
- Van der Werf G.W.,Sass-Klaassen U.G.W., and Mohren G.M.J., 2007. The impact of the 2003 summer drought on the intra-annual growth pattern of beech (Fagus sylvatica L.) and oak (Quercus robur L.) on a dry site in the Netherlands. Dendrochronologia 25: 103–112. [CrossRef] [Google Scholar]
- Warren C.R., McGrath J.F., and Adams M.A., 2001. Water availability and carbon isotope discrimination in conifers. Oecologia 127: 476–486. [CrossRef] [PubMed] [Google Scholar]
- Zweifel R., Zimmermann L., Zeugin F., and Newbery D.M., 2006. Intra-annual radial growth and water relations of trees: Implications towards a growth mechanism. J. Exp. Bot. 57: 1445–1459. [CrossRef] [PubMed] [Google Scholar]
All Tables
Summary of some key climatic parameters recorded during the growing period of the summer green Q. pubescens from 2004 to 2006. T max and T min denote recorded maximum and minimum air temperatures, respectively. Monthly and total (SUM) rainfall is shown in mm from April to October.
All Figures
 |
Figure 1 Representative cross-section with collapsed cell rows (indicated by white arrows) in the LW of stressed oaks. Differentiation in EW, LW and newly formed LW after severe drought stress (collapsed cells) is indicated on the lefthand scale. Red and blue coloured cell walls indicate dead (lignified) and living (non-lignified) cells, respectively. The white bar represents 1 mm. (A color version of this figure is available at www.afs-journal.org.) |
| In the text | |
 |
Figure 2 Annual changes of ring width in well-watered (control; closed symbols) and drought-stressed (stress; open symbols) trees. Means and standard errors of control and drought-stressed trees are shown. Asterisks indicate significant differences between stress and corresponding control values (p ≤ 0.05). Insert depicts increment of radial stem growth since 2003. |
| In the text | |
 |
Figure 3 Annual changes in wood characteristics in EW (circles) and LW (triangles) of control (closed symbols) and stressed oaks (open symbols) since 2003. NV, MVA, TVA and CA denote number of vessels, mean vessel area, total vessel area and conduit area, respectively. Means and standard errors of at least four trees per treatment are shown as a percentage of the starting situation (control) in 2003. Asterisks indicate significant differences between stress and corresponding control values (p ≤ 0.05). |
| In the text | |
 |
Figure 4 Changes in carbon isotope composition (δ13C) in tree rings from 2003 to 2006. Means and standard errors of at least four trees per treatment are shown. Asterisks indicate significant differences between stress and corresponding control values (p ≤ 0.05). |
| In the text | |
 |
Figure 5 Changes in carbon isotope composition (δ13C) in EW and LW from 2003 to 2006. Means and standard errors of at least four trees per treatment are shown. Asterisks indicate significant differences between stress and corresponding control values (p ≤ 0.05). |
| In the text | |


