| Issue |
Ann. For. Sci.
Volume 67, Number 4, June 2010
|
|
|---|---|---|
| Article Number | 405 | |
| Number of page(s) | 9 | |
| DOI | https://doi.org/10.1051/forest/2009125 | |
| Published online | 11 March 2010 | |
Original article
Mycorrhization, growth and nutrition of Pinus halepensis seedlings fertilized with different doses and sources of nitrogen
Mycorhization, croissance et nutrition de semis de Pinus halepensis fertilisés avec différentes doses et sources d’azote
1
Depto. Biología Aplicada, Botánica, Univ. Miguel Hernández, Avda. Universidad s/n
03202, Elche Alicante,
Spain
2
Depto. Biología Vegetal, Botánica, Univ. Murcia, Campus Espinardo, 30100
Murcia,
Spain
* Corresponding author: gdiaz@umh.es
Received:
22
June
2009
Accepted:
9
September
2009
• Although fertilization is commonly used in nurseries, the effects of high level of nitrogen on Pinus halepensis mycorrhization are still unknown.
• The effect of fertilization at different N levels (low-LN: 35 mg/plant; medium-MN: 60 mg/plant; high-HN: 120 mg/plant), differing N sources (ammonium-(NH4)2SO4; nitrate-HNO3; ammonium+nitrate-NH4NO3) and inoculation with Pisolithus tinctorius and Lactarius deliciosus on the mycorrhization, growth and nutrient status of P. halepensis has been studied.
• P. tinctorius 3SR showed higher mycorrhizal ability (100% of mycorrhizal seedlings) than L. deliciosus (nearer to 50%). The application of increasing doses of N resulted in a significant reduction of mycorrhizal seedlings but no differences were observed between NH4 and NO3 as N source at the 60 mg N/plant dose applied. The effects of fertilization on growth were mainly observed in uninoculated plants. The use of NH4 increased growth in non-mycorrhized plants. Nutrient status was similar in all cases except for K concentration, which was higher in plants mycorrhized with P. tinctorius. Interactions between inoculation and fertilization were found, mycorrhizal effects appearing only at LN fertilization.
• It is advisable to avoid high doses of N fertilization in order to produce mycorrhizal P. halepensis seedlings.
Résumé
• Bien que la fertilisation soit couramment utilisée dans les pépinières, les effets d’un niveau élevé d’azote sur la mycorhization chez Pinus halepensis sont encore inconnus.
• L’effet de la fertilisation à différents niveaux d’azote (LN bas: 35 mg/plant ; MN moyen : 60 mg/plant ; HN élevé : 120 mg/plant), avec différentes sources d’azote (ammonium-(NH4)2SO4 ; nitrate-HNO3; ammonium+nitrate-NH4NO3) et inoculation avec Pisolithus tinctorius et Lactarius deliciosus a été étudié pour la mycorhization, la croissance et l’état nutritionnel du Pinus halepensis.
• P. tinctorius 3SR a montré une aptitude plus élevée à la mycorhization (100 % des plants mycorhizés) que L. deliciosus (proche de 50 %). L’application de doses croissantes d’azote a entraîné une réduction significative des semis mycorhizés mais aucune différence n’a été observée entre NH4 et NO3 comme source de N à une dose appliquée de 60 mg N/plant. Les effets de la fertilisation sur la croissance ont été principalement observés chez les plants non inoculés. L’utilisation de NH4 augmente la croissance chez les plants non mycorhizés. L’état nutritionnel a été similaire dans tous les cas sauf pour la concentration de K, qui est plus élevée chez les plants mycorhizés par P. tinctorius. Des interactions entre inoculation et fertilisation ont été trouvées, les effets mycorhiziens n’apparaissant qu’à des fertilisations LN.
• Il est conseillé d’éviter de fortes doses de fertilisation azotée pour produire des plants de P. halepensis mycorhizés.
Key words: mycorrhiza / nitrogen / Pinus halepensis / fertilization / nursery
Mots clés : mycorhize / azote / Pinus halepensis / fertilisation / pépinière
© INRA, EDP Sciences, 2010
1. INTRODUCTION
Several crop-related factors intervene in the production process of quality forest plants at the commercial level of which fertilization is one of the most critical and, if suitably adjusted, will produce plants with an optimum nutritional status to be subsequently transplanted in the field. Nitrogen (N) is the nutrient mostly consumed by plants and it largely limits the growth of plants growing in containers (Landis et al., 1989). Fertilization programmes are initially based on the amount of N provided as it forms part of many vital compounds for plant development (i.e., chlorophyll, amino acids and proteins) and is essential for the development of healthy leaves.
It is generally accepted that inoculation with mycorrhizal fungi is an advisable practice for producing high quality nursery seedlings. Mycorrhizal symbiosis may improve the quality of seedlings by increasing plant growth and/or their physiological attributes (Brundrett et al., 1996). These benefits relate to the uptake of water and nutrients, enhanced root enzyme activity, a more efficient use of water, a higher photosynthesis rate or greater protection against pathogens. Then, mycorrhized seedlings are expected to overcome outplanting stress in comparison with non-mycorrhizal plants (Luo et al., 2009; Zhu et al., 2008). This is especially important as far as Pinus halepensis Miller is concerned, one of the most planted pine species in the Mediterranean basin. This species plays a critical role in the restoration of degraded lands under adverse climate and soil conditions. Several studies report the beneficial effect of mycorrhizal inoculation on field performance in P. halepensis (Díaz et al., 2004; Parladé et al., 2004; Querejeta et al., 1998; Rincón et al., 2007a; Roldan et al., 1996).
Nonetheless, the N fertilization regime may influence mycorrhizae development. High N levels are commonly used in nurseries to produce container-grown plants. However, a good number of studies have shown that high N concentrations in substrates inhibit ectomycorrhizae development (Arnebrant, 1994; Brunner and Brodbeck, 2001; Holopainen and Heinonen-Tanski, 1993; Wallander and Nylund, 1991) whereas a moderate deficiency of N favours mycorrhization.
Not all mycorrhizal fungi species show the same sensitivity to N fertilization; some species are highly sensitive to excessive nitrogen, others colonize nitrogen-rich substrates (Wallander, 1994; Wallander and Nylund, 1992).
Another important aspect of N fertilization is the source used since the composition of the fertilizer determines whether N is assimilated and its effect on the plant. Plants can absorb N as ammonium (NH4) or nitrate (NO3), and balanced formulae of both N sources are usually employed (Landis et al., 1989). Some authors have observed how mycorrhizal development is more affected when NO3 rather than NH4 is used as N source (Väre, 1989). In other cases, NH4 seems more harmful than NO3 (Termoshuizen and Ket, 1991; Wallander and Nylund, 1991).
Therefore, it is important to adjust N fertilization when producing quality mycorrhizal plants in nurseries. Although former works have studied the influence of fertilization on the mycorrhization of several forest species, no information about P. halepensis is available. The objective of this work was to determine the effect of N fertilization in relation to both the source and dose used on plant growth, nutrient status and mycorrhization of P. halepensis inoculated with three strains of ectomycorrhizal fungi and to asses the effect of mycorrhizae on plant growth attributes.
2. MATERIALS AND METHODS
2.1. Plant material
The container used was a Poliforest®, Poliex, Spain tray made of expanded polystyrene with 25 individual cells filled with a 350 cc, plastic, removable and openable pot. It has vertical ribs to prevent spiralling and an open base to allow for drainage. The potting substrate used was unsterilized Sphagnum peat VAPO® BO, Finland (pH 5.3). P. halepensis seeds collected from Maestrazgo, Los Serranos, Teruel, Spain were surface disinfected by shaking in 30 vol H2O2 for 20 min., then rinsed in distilled water and sown in the container (3–4 seeds per cell) on February. The containers were placed in the glasshouse (Ta ranged from 6 to 25 °C). Germination occurred within 20–30 d. After germination, seedlings were cleared to one per cell. Plants were moved outdoors and grown from March to November under natural climatic and day/night conditions, shaded by a mesh (40% radiation reduction) in the summer.
2.2. Fungal inoculum and inoculation
The inoculated fungal species were Pisolithus tinctorius (Pers.) Coker & Couch (strains 3SR, collected at Uceda, Guadalajara, Spain under a Quercus rotundifolia-P. halepensis stand and Mx, collected at Tlaxcala, Mexico under P. oocarpa) and Lactarius deliciosus (L. ex Fr.) Gray (strain LDF5, collected at Valencia, Spain under a P. halepensis stand).
Isolations of mycorrhizal strains were done with explants from basidioma tissue on modified Melin-Norkrans medium (MMN) (Marx, 1969) for P. tinctorius, and according to the procedure described in Díaz et al. (2009) for L. deliciosus. They were transferred to fresh media every three months. Reference cultures were deposited at the culture collection of Laboratory of Mycology-Mycorrhizas of the University of Murcia, Spain.
Inoculum of P. tinctorius was produced using 1 L flasks filled with a sterilized (120 °C, 20 min) mixture of peat and vermiculite (1:4 v/v) moistened with MMN liquid medium. Then, flasks were inoculated with several plugs of mycelium growing on MMN solid agar plates, and incubated at 23 °C in the dark for approximately 8 weeks. Inoculum of L. deliciosus was prepared by growing mycelia in flasks with MMN liquid medium and incubation at 23 °C in the dark for 4–8 weeks due to the poor development of the mycelium on solid substrate. Inocula were checked for viability on MMN agar plates before use.
Seedlings were inoculated in the spring, three months after emergence. According to previous experiments (unpublished data), 25 mL/plant of peat-vermiculite inoculum were placed onto the root surface for P. tinctorius treatments, and 10 mL/plant of liquid inoculum were injected in the root zone for the L. deliciosus treatment. Control plants remained uninoculated.
2.3. Experimental design
Two experiments were done independently at the forest nursery from Centro Nacional de Mejora Genética Forestal El Serranillo, of the Spanish Ministerio de Medio Ambiente y Medio Rural y Marino, at Guadalajara, Spain.
Experiment 1: A factorial experiment was set up to check the effect of two factors: (1) application of different N fertilization levels (low-LN, medium-MN and high-HN, corresponding to 35, 60 and 120 mg N/plant, respectively) and (2) inoculation with mycorrhizal fungi (P. tinctorius 3SR, P. tinctorius Mx, L. deliciosus, uninoculated control) on mycorrhizal development and plant growth. There were 100 replicates per treatment.
All the plants were fertilized every two weeks from April to September except August with Peter’s Professional®, Scotts, Spain fertilizer with different N-P-K formulations suitable for plant development at each plant growth phase: Conifer Starter (7-40-17) at germination (twice), Conifer Grower (20-7-19) during plant growth (6 times) and Conifer Finisher (4-25-35) at hardening (twice). Plants with the MN and HN levels were supplemented with 10 mL/plant of a solution of NH4NO3 at the adequate concentration. This supplement was applied 6 times coinciding with fertilization at the plant growth phase. The total amount of macronutrients received by seedlings with the LN, MN and HN treatments was 35-27-61, 60-27-61 and 120-27-61 mg NPK/plant, respectively.
Experiment 2: A factorial experiment was set up to check the effect of two factors: (1) application of different N sources (NH4, NO3, NH4 + NO3) and (2) inoculation with mycorrhizal fungi (P. tinctorius 3SR, L. deliciosus, uninoculated control) on mycorrhizal development and plant growth. There were 100 seedlings per treatment. Plants were fertilized with Peter’s Professional® fertilizer with the LN treatment schedule of Experiment 1. N source treatments were supplied as a solution of 21% (NH4)2SO4, 59% HNO3 and 45% NH4NO3. These supplements were applied 6 times coinciding with fertilization at the plant growth phase. The total amount of macronutrients received by seedlings was 60-27-61 mg NPK/plant.
2.4. Measurements and statistical analysis
All the seedlings were assessed for mycorrhizal development at five months post-inoculation. Two parameters were determined: (1) percentage of mycorrhizal seedlings (ratio between seedlings that became mycorrhizal by the inoculated fungi and total inoculated seedlings for each treatment) and (2) mycorrhizal colonization index (percentage of mycorrhizal colonization by the inoculated fungi in each root system determined by a non-destructive, visually-determined observation and expressed as an index ranging from 0 to 5 (0: 0%, 1: 1–20%, 2: 21–40%, 3: 41–60%, 4: 61–80%, 5: 81–100% of the root system colonized by mycorrhiza) (Abourouh, 1996).
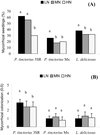 |
Figure 1 Percentages of mycorrhizal seedlings (A) and mycorrhizal colonization index (B) on P. halepensis inoculated with three ectomycorrhizal fungi under different N fertilization levels (low-LN: 35 mg/plant, medium-MN: 60 mg/plant, high-HN: 120 mg/plant). For each inoculation treatment, different letters indicate significant differences (Contingency tables for percentage of mycorrhizal seedlings, Duncan’s test, p ≤ 0.05 for mycorrhizal colonization). |
All the seedlings were measured for height and root collar diameter. Twenty-five seedlings were randomly selected from each treatment. Growing media was removed from the roots. Plant fractions (shoots, roots) were separated, washed, dried at 60 °C for 48 h, and weighed. Needles were analyzed for nutrient contents: N by the Kjeldahl method, P by colorimetry (Olsen, 1954) and K by atomic absorption spectroscopy.
Data were analyzed with the software package SPSS 10.0 for Windows. The percentages of mycorrhizal seedlings for each treatment were analyzed by contingency tables. Mycorrhizal colonization, growth and nutrition data were analyzed by a two-way ANOVA to see the effects of the factors. Significant differences among treatments were determined by Duncan’s multiple range test. The mycorrhizal colonization data were arc-sin transformed before performing ANOVAs to achieve normality.
3. RESULTS
3.1. Mycorrhiza formation
The three fungal strains inoculated were able to form mycorrhizas on P. halepensis, but showed a different mycorrhizal capacity. The autochthonous P. tinctorius 3SR was much more effective (with almost 100% of mycorrhizal seedlings) than P. tinctorius from Mexico (18–25% of mycorrhizal seedlings). L. deliciosus formed around 50% of mycorrhizal seedlings. However, the percentage of mycorrhizal seedlings obtained was affected by the amount and source of N applied.
Applying increasing doses of N significantly reduced mycorrhizal seedlings when compared with the values obtained with low doses. Whereas mycorrhiza formation by P. tinctorius 3SR was only affected at the highest N dose, P. tinctorius Mx and L. deliciosus were affected at the MN and HN doses (Fig. 1A). Mycorrhizal colonization was not affected by fertilization treatments, except for P. tinctorius 3SR which achieved a higher mycorrhizal colonization at the LN level than at the MN or HN levels (Fig. 1B).
The mycorrhizal seedlings rates obtained with NH4NO3 were lower than those obtained with NH4 or NO3 as N source (Fig. 2A). Mycorrhizal colonization was slightly lower for P. tinctorius 3SR with NH4, and was similar for L. deliciosus with the three N sources (Fig. 2B).
 |
Figure 2 Percentages of mycorrhizal seedlings (A) and mycorrhizal colonization index (B) on P. halepensis inoculated with three ectomycorrhizal fungi under different N fertilization sources (ammonium nitrate-NH4NO3, ammonium-(NH4)2SO4, nitrate-HNO3). For each inoculation treatment, different letters indicate significant differences (Contingency tables for percentage of mycorrhizal seedlings, Duncan’s test, p ≤ 0.05 for mycorrhizal colonization). |
Spontaneous mycorrhizae formed by other fungi such as Rhizopogon or Suillus were rarely observed. Indeed, mycelia and strands of Telephora terrestis appeared at the bottom part of root systems at the end of the experiment but apparently they did not prevent colonization by the inoculated fungi.
3.2. Plant growth and nutrition
Inoculation and N dose factors had a significant effect on almost all the plant growth parameters. It is important to note that significant interactions between inoculation and N dose factors were found for all plant growth parameters (Tab. I).
Plant growth parameters on P. halepensis seedlings inoculated with three ectomycorrhizal fungi under different N fertilization levels (low-LN: 35 mg/plant, medium-MN: 60 mg/plant, high-HN: 120 mg/plant). For each inoculation treatment, different minor letters indicate significant differences among fertilizations. For each N level, different capital letters indicate significant differences among inoculation treatments (Duncan’s test, p ≤ 0.05).
The effect of fertilization on plant functional attributes varied under the different inoculation treatments. Fertilization with increasing doses of N significantly increased the shoot dry weight of uninoculated plants and plants inoculated with P. tinctorius Mx, and the shoot/root ratio of L. deliciosus treatments. With P. tinctorius 3SR however, the plant growth parameters were higher with the lowest N dose. The comparison of inoculation treatments within each fertilization level shows that plants mycorrhized with P. tinctorius were higher and had a larger diameter than uninoculated plants with lower N doses (Tab. I). No significant differences were observed among treatments for nutrient concentrations; only the K concentration was affected by N dose and inoculation factors (Tab. II).
Nutrient concentration in needles of P. halepensis seedlings inoculated with three ectomycorrhizal fungi under different N fertilization levels (low-LN: 35 mg/plant, medium-MN: 60 mg/plant, high-HN: 120 mg/plant). For each inoculation treatment, different letters indicate significant differences (Duncan’s test, p ≤ 0.05).
The effects of inoculation and N source factors on plant growth are shown in Table III. The inoculation factor was only significant for root dry weight, whereas the N source significantly influenced height, diameter, branches and root dry weight. Significant interactions were found for height and shoot dry weight. The growth parameters slightly increased when the N source was NH4, especially with uninoculated plants. Height and the number of branches were higher in P. tinctorius 3SR seedlings fertilized with NH4. No differences were observed for N and P concentrations among treatments; inoculation and N source were significant only for K (Tab. IV).
Plant growth parameters on P. halepensis seedlings inoculated with three ectomycorrhizal fungi under different N fertilization sources (ammonium nitrate-NH4NO3,ammonium-(NH4)2SO4, nitrate-HNO3). For each inoculation treatment, different minor letters indicate significant differences among fertilizations; For each N source, different capital letters indicate significant differences among inoculation treatments (Duncan’s test, p ≤ 0.05).
Nutrient concentration in needles of P. halepensis seedlings inoculated with three ectomycorrhizal fungi under different N fertilisation sources (ammonium nitrate-NH4NO3,ammonium-(NH4)2SO4, nitrate-HNO3). For each inoculation treatment, different letters indicate significant differences (Duncan’s test, p ≤ 0.05).
4. DISCUSSION
4.1. Effect of N fertilization on mycorrhizal development
The high mycorrhizal capacity shown by P. tinctorius 3SR makes this strain a good candidate for nursery inoculations with P. halepensis. P. tinctorius has been widely used for inoculating several conifer species in nurseries with varying success rates (Brundrett et al., 1996; Marx, 1981; Rincón et al., 2005). The effectiveness of fungus L. deliciosus was only around 50%, which is in line with other previous reports for this fungus (Gonzalez-Ochoa et al., 2003; Parladé et al., 2003). This low mycorrhizal capacity may not be attributed to the type of inoculum used, since inoculum as a mycelial suspension in a liquid carrier has been previously shown to be most effective for this strain (Díaz et al., 2009). Thus it would probably be necessary to optimize some cultural or environmental factors to achieve higher mycorrhizal rates in nurseries.
Increasing N fertilization negatively affected the mycorrhizal capacity of the fungi used, which is in agreement with other studies done on different plant and fungal species (Arnebrant, 1994; Holopainen and Heinonen-Tanski, 1993). An excess N input has been demonstrated to reduce the fungal biomass (Wallander and Nylund, 1991; 1992). According to the carbohydrate theory, high N availability implies consumption of carbohydrate to reduce NO3 to NH4 inside the roots. This process reduces the pool of sugar concentration in the roots to reach levels that are too low to initiate infection. So N assimilation acts like a carbon sink (Nehls, 2004).
In relation to N sources used, the number of mycorrhizal plants was similar with NH4 and NO3. It is interesting to point out that the data obtained with the three treatmentes were comparable with those obtained at the same N dose in Experiment 1. Previous reports have documented that ectomycorrhizal fungi prefer NH4 to NO3 (Guidot, 2005; Rangel-Castro et al., 2002) as the latter has a strong inhibitory effect on mycorrhizal development (Väre, 1989). Other authors found that NH4 affected mycorrhizae more negatively than NO3 (Termoshuizen and Ket, 1991; Wallander and Nylund, 1991). It is likely that the amount applied (60 mg N/plant) is not enough to detect inhibitory effects. Therefore, the N dose factor appears to be more critical than the N source factor.
Although the use of NH4NO3 reduced mycorrhiza formation, it maintained sufficient high levels of mycorrhization. Therefore, it may be considered a compatible fertilizer with nursery inoculation if the recommendations to use balanced formulae of both N sources are followed (Domínguez, 1997; Landis, 1989).
The mycorrhizal colonization of P. tinctorius 3SR inside the roots is affected by N, unlike the other fungi used. Previously, P. tinctorius was found to be sensitive to high N fertilization (Rincón et al., 2007b). Tolerance to N may correspond to a distinct enzymatic activity that implies a different substrate exploitation method (Taniguchi et al., 2008) and could be one of the factors regulating the distribution of ECM fungi in poor or rich N forests. Wallander (1994) suggested that species which rapidly absorb N and swiftly transfer it to the host plant may be more sensitive to excess N because they tend to use large amounts of carbohydrates while assimilating this element. This, in turn, reduces the available amount of carbohydrates for fungal growth.
4.2. Effect of N fertilization on plant growth and nutrition
The general tendency of fertilization to increase shoot weight and the shoot/root (S/R) ratio has been observed with some treatments of this study. This response has been previously documented for several plant species (Canham et al., 1996), which also include Mediterranean species (Oliet et al., 2004; Villar-Salvador et al., 2004; 2005). The S/R ratio is interesting although controversial for field survival, particularly when availability of water is restricted. Plants with a high S/R ratio transpire more than plants with a low S/R ratio, which may increase their drought vulnerability to soil water shortage after outplanting. However, there is evidence that P. halepensis plants with a high S/R ratio display greater field survival in some experiments than small seedlings with low S/R rates (Oliet et al., 2009). As high N fertilization is generally recommended for seedling production for afforestation purposes (Puertolas et al., 2003), it is advisable to check sufficiently high N concentrations for plant growth that enable adequate mycorrhization.
Plant growth attributes increased more with NH4 than with NH4NO3.Under the typical high humidity and substrate porosity nursery conditions, NH4NO3 will partially be lost through leaching, whereas NH4 forms leach very little. This may account for the improved assimilation of NH4 as certain parameters indicate. However, the use of NH4 as an exclusive N source as a fertilizer is not recommended for container-grown forest plants.
The N and P concentration was similar in all cases irrespectively of the treatment applied, and their levels remain within the range of values that are considered acceptable for container-grown P. halepensis (Puértolas et al., 2003). Although N fertilization has been shown to increase tissue N concentration (Oliet et al., 2004; Villar-Salvador et al., 2005), this effect was not observed in our study. It is likely due to the differences in the N amounts applied not being large enough to be reflected in shoot content. Indeed, shoot growth seems to increase in parallel to that of N uptake, thus producing a dilution effect. The total N content per plant in uninoculated seedlings was significantly higher at the highest dose (1.57 mg N/plant) than at the low (1.29 mg N/plant) or medium (1.30 mg N/plant) dose.
4.3. Effect of inoculation on plant growth and nutrition
Inoculation with mycorrhizal fungi did not produce generalized but sporadic effects on plant morphological attributes, which depended on the N fertilization dose and source. Negative inoculation x fertilization interactions were found, so mycorrhizal effects appeared only at low fertilization, whereas high fertilization eliminated these effects. Interactive effects between fertilization and mycorrhization are frequent (Hilszanska et al., 2008; Parladé et al., 2003; Rincón et al., 2005; 2007b; Smith and Read, 1997) and are attributed to the large amount of carbohydrates that the fungus requires to establish symbiosis (Dosskey et al., 1991). This justifies the detrimental effects of the mycorrhiza observed, particularly on the P. tinctorius 3SR strain which reached the highest root colonization levels.
Growth data are reflected in the nutrient data and only effects on K concentration were observed. The K concentration of plants mycorrhized with P. tinctorius was higher than in uninoculated plants. The K concentration in tissues relates to the vitality of the nursery plant and resistance to fungal-related diseases. It plays a key role in osmotic adjustment, in regulating the stomatic aperture and contributes to reduce losses caused by transpiration (Landis, 1989). Therefore, mycorrhization with this fungus may be advantageous for the plant as it is likely to be more resistant to drought, thus ensuring a higher post-transplant survival rate.
In this work, N fertilization affected the amount and scope of mycorrhizae with P. tinctorius and L. deliciosus in P. halepensis. The influence of mycorrhizae on seedling growth was modest and depended on the dose and source of N. Therefore, it is advisable to adjust N fertilization, avoiding too high doses, in order to produce mycorrhizal seedlings to be outplanted.
Acknowledgments
This study was supported by the agreement between ICONA-Instituto para la conservación de la Naturaleza, Spain and the University of Murcia. We are grateful to J.L. Peñuelas, P. Villar and S. Domínguez from Centro Nacional de Mejora Genética y Forestal El Serranillo, Guadalajara, Spain, for their helpful comments and suggestions. We are also grateful to Helen Warburton for her help with the English translation.
References
- Abourouh M., 1996. Les évaluations quantitatives des mycorhizes en pépinière et sur le terrain. Cah. Options méditerr. 20: 51–61. [Google Scholar]
- Arnebrant K., 1994. Nitrogen amendments reduce the growth of extramatrical ectomycorrhizal mycelium. Mycorrhiza 5: 7–15. [CrossRef] [Google Scholar]
- Brunner I. and Brodbeck S., 2001. Response of mycorrhizal Norway spruce seedlings to various nitrogen loads and sources. Environ. Pollut. 114: 223–233. [CrossRef] [PubMed] [Google Scholar]
- Brundrett M., Bougher N., Dell B., Grove T. and Malajczuk N., 1996. Working with mycorrhizas in forestry and agriculture, ACIAR Monograph 32, Camberra, Australia, 374 p. [Google Scholar]
- Canham C.D., Berkowitz A.R., Kelly V.R., Lovett G.M., Ollinger S.F. and Schnurr J., 1996. Biomass allocation and multiple resource limitation in tree seedlings. Can. J. For. Res. 26: 1521–1530. [CrossRef] [Google Scholar]
- Díaz G., Carrillo C. and Honrubia M., 2009. Production of Pinus halepensis seedlings inoculated with the edible fungus Lactarius deliciosus under nursey conditions. New For. 38: 215–227. [CrossRef] [Google Scholar]
- Díaz G., Gutiérrez A. and Honrubia M., 2004. Utilización de micorrización controlada en la reforestación de un suelo agrícola con pino carrasco. Cuad. Soc. Esp. Cien. For. 17: 151–155. [Google Scholar]
- Dominguez A., 1997. Tratado de Fertilización. Mundi-Prensa, Madrid, 613 p. [Google Scholar]
- Doskey M.G., Boersman L. and Linderman R.G., 1991. Role for the photosynthate demand of ectomycorrhizas in response of Douglas-fir seedlings to drying soil. New Phytol. 117: 327–334. [CrossRef] [Google Scholar]
- González-Ochoa A.I., de las Heras J., Torres P. and Sánchez-Gómez E., 2003. Mycorrhization of Pinus halepensis Mill. and Pinus pinaster Aiton seedlings in two commercial nurseries. Ann. For. Sci. 60: 43–48. [CrossRef] [EDP Sciences] [Google Scholar]
- Guidot A., Verner A.C., Debaud J.C. and Marmeisse R., 2005. Intraspecific variation in use of different organic nitrogen sources by the ectomycorrhizal fungus Hebeloma cylindrosporum. Mycorrhiza 15: 167–177. [CrossRef] [PubMed] [Google Scholar]
- Hilszczanska D., Malecka M. and Sierota Z., 2008. Changes in nitrogen level and mycorrhizal structure of Scots pine seedlings inoculated with Thelephora terrestris. Ann. For. Sci. 65: 409. [CrossRef] [EDP Sciences] [Google Scholar]
- Holopainen T. and Heinonen-Tanski H., 1993. Effects of different nitrogen sources on the growth of Scots pine seedlings on the ultraestructure and development of their mycorrhizae. Can. J. For. Res. 23: 362–372. [CrossRef] [Google Scholar]
- Landis T.D., Tinus R.W., McDonald S.E. and Barnett J.P., 1989. The container tree nursery manual, vol. 4, Mineral nutrients and fertilization. Agric. Hanbook 674, USDA Forest Service, Washington D.C., 120 p. [Google Scholar]
- Luo Z., Li K., Jiang X. and Polle A., 2009. Ectomycorrhizal fungus (Paxillus involutus) and hydrogel affect performance of Populus euphratica exposed to drought stress. Ann. For. Sci. 66: 106. [CrossRef] [EDP Sciences] [Google Scholar]
- Marx D.H., 1969. The influence of ectotrophic mycorrhizal fungi on the resistance of pine roots to pathogenic infections. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology 59: 153–163. [Google Scholar]
- Marx D.H., 1981. Variability in ectomycorrhizal development and growth among isolates of Pisolithus tinctoriusas affected by source, age and reisolation. Can J. For. Sci. 11: 168–174. [Google Scholar]
- Ne’eman G. and Trabaud L., 2000. Ecology, biogeography and management of Pinus halepensis andP. brutia forest ecosystems in the Mediterranean Basin. Backhuys Publishers, The Netherlands, 407 p. [Google Scholar]
- Nehls U., 2004. Carbohydrates and nitrogen: Nutrients and signals in ectomycorrhizs. In: Varma A., Abbott L., Werner D. and Hampp R. (Eds.), Plant surface microbiology, Springer Verlag, Berlin Heidelberg, Germany, pp. 377–392. [Google Scholar]
- Oliet J., Planelles R., Segura M.L., Artero F. and Jacobs D.F., 2004. Mineral nutrition and growth of containerized Pinus halepensis seedlings under controlled-release fertilizer. Sci. Hort. 103: 113–129. [Google Scholar]
- Oliet J., Planelles R., Artero F., Valverde R., Jacobs D.F. and Segura M.L., 2009. Field performance of Pinus halepensis planted in Mediterranean arid conditions: relative influence of seedling morphology and mineral nutrition. New For. 37: 313–331. [CrossRef] [Google Scholar]
- Olsen S.R., Cole C.V., Watanabe F.S. and Dean L.A., 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circ. 939. [Google Scholar]
- Parladé J., Luque J., Pera J. and Rincón A. 2004. Field performance of Pinus pineaandPinus halepensis seedlings inoculated with Rhizopogon spp. and out-planted in formerly arable land. Ann. For. Sci. 61: 504–514. [Google Scholar]
- Parladé J., Pera J. and Luque J., 2003. Evaluation of mycelial inocula of edible Lactarius species for the production of Pinus pinaster and P. sylvestris mycorrhizal seedlings under greenhouse conditions. Mycorrhiza 14: 171–176. [PubMed] [Google Scholar]
- Puértolas J., Gil. L. and Pardos J.A., 2003. Effects of nutritional status and seedling size on field performance of Pinus halepensis planted on former arable land in the Mediterranean basin. Forestry 76: 159–168. [CrossRef] [Google Scholar]
- Querejeta J.I., Roldán A., Albadalejo J. and Castillo V., 1998. The role of mycorrhizae, site preparation, and organic amendment in the afforestation of a semi-arid mediterranean site with Pinus halepensis. For. Sci. 44: 203–211. [Google Scholar]
- Rangel-Castro J.I., Danell E. and Taylor A.F., 2002. Use of different nitrogen sources by the edible ectomycorrhizal mushroom Cantharellus cibarius. Mycorrhiza 12: 131–137. [CrossRef] [PubMed] [Google Scholar]
- Rincón A., Parladé J. and Pera J., 2005. Effects of ectomycorrhizal inoculation and the type of substrate on mycorrhization, growth and nutrition of containerised Pinus pinea L. seedlings produced in a commercial nursery. Ann. For. Sci. 62: 1–6. [EDP Sciences] [Google Scholar]
- Rincón A., de Felipe M.R. and Fernández Pascual M., 2007. Inoculation of Pinus halepensis Miller with selected ectomycorrhizal fungi improves seedling establishemnt 2 years after planting in a degraded gypsum soil. Mycorrhiza 18: 23–32. [CrossRef] [PubMed] [Google Scholar]
- Rincón A., Parladé J. and Pera J., 2007. Influence of the fertilisation method in controlled ectomycorrhizal inoculation of two Mediterranean pines. Ann. For. Sci. 64: 577–783. [CrossRef] [EDP Sciences] [Google Scholar]
- Roldán A., Querejeta J.I., Albadalejo J. and Castillo V. 1996. Growth response of Pinus halepensis to inoculation with Pisolithus arhizus in a terraced rangeland amended with urban refuse. Plant Soil 179: 35–43. [CrossRef] [Google Scholar]
- Smith S.E. and Read D.J., 1997. Mycorrhizal symbiosis. Academic Press, Cambridge, 605 p. [Google Scholar]
- Taniguchi T., Kataoka R. and Futai K., 2008. Plant growth and nutrition in pine (Pinus thunbergii) seedlings and dehydrogenase and phosphatase activity of ectomycorrhizal root tips inoculated with seven individual ectomycorrhizal fungal species at high and low nitrogen conditions. Soil. Biol. Biochem. 40: 1235–1243. [CrossRef] [Google Scholar]
- Termoshuizen A.J. and Ket P.C., 1991. Effects of NH4 and nitrate on mycorrhizal seedlings ofPinus sylvestris. Eur. J. For. Pathol. 21: 404–413. [CrossRef] [Google Scholar]
- Väre H., 1989. Effect of nitrogen on the growth ofSuillus variegatus on mycorrhizal and non-mycorrhizal Pinus sylvestris seedlings. Aquilo. Ser. Bot. 26: 19–24. [Google Scholar]
- Villar-Salvador P., Planelles R., Enríquez E. and Peñuelas Rubira J., 2004. Nursery cultivation regimes, plant functional attributes, and field performance relatioships in the Mediterranean oak Quercus ilex L. For. Ecol. Manage. 196: 257–266. [CrossRef] [Google Scholar]
- Villar-Salvador P., Puértolas J., Peñuelas J.L. and Planelles R., 2005. Effect of nitrogen ferillization in the nursery on the drought and frost resistance of Mediterranean forest species. Investig. Agrar. Sist. Recur. For. 14: 408–418. [Google Scholar]
- Wallander H., 1994. A new hypothesis to explain allocation of dry matter between mycorrhizal fungi and pine seedlings in relation to nutrient supply. Plant Soil 168: 243–248. [CrossRef] [Google Scholar]
- Wallander H. and Nylund J.E., 1991. Effects of excess nitrogen on carbohydrate concentration and mycorrhizae development of Pinus sylvestris L. seedlings. New Phytol. 119: 405–441. [CrossRef] [Google Scholar]
- Wallander H. and Nylund J.E., 1992. Effects of excess nitrogen and phosphorous starvation on the extramatrical mycelium of ectomy-corrhizas of Pinus sylvestris L. New Phytol. 120: 495–503. [CrossRef] [Google Scholar]
- Zhu J., Li F., Xu M., Kang H. and Wu X., 2008. The role of ectomycorrhizal fungi in alleviating pine decline in semiarid sandy soil of northern China: an experimental approach. Ann. For. Sci. 65: 304. [CrossRef] [EDP Sciences] [Google Scholar]
All Tables
Plant growth parameters on P. halepensis seedlings inoculated with three ectomycorrhizal fungi under different N fertilization levels (low-LN: 35 mg/plant, medium-MN: 60 mg/plant, high-HN: 120 mg/plant). For each inoculation treatment, different minor letters indicate significant differences among fertilizations. For each N level, different capital letters indicate significant differences among inoculation treatments (Duncan’s test, p ≤ 0.05).
Nutrient concentration in needles of P. halepensis seedlings inoculated with three ectomycorrhizal fungi under different N fertilization levels (low-LN: 35 mg/plant, medium-MN: 60 mg/plant, high-HN: 120 mg/plant). For each inoculation treatment, different letters indicate significant differences (Duncan’s test, p ≤ 0.05).
Plant growth parameters on P. halepensis seedlings inoculated with three ectomycorrhizal fungi under different N fertilization sources (ammonium nitrate-NH4NO3,ammonium-(NH4)2SO4, nitrate-HNO3). For each inoculation treatment, different minor letters indicate significant differences among fertilizations; For each N source, different capital letters indicate significant differences among inoculation treatments (Duncan’s test, p ≤ 0.05).
Nutrient concentration in needles of P. halepensis seedlings inoculated with three ectomycorrhizal fungi under different N fertilisation sources (ammonium nitrate-NH4NO3,ammonium-(NH4)2SO4, nitrate-HNO3). For each inoculation treatment, different letters indicate significant differences (Duncan’s test, p ≤ 0.05).
All Figures
 |
Figure 1 Percentages of mycorrhizal seedlings (A) and mycorrhizal colonization index (B) on P. halepensis inoculated with three ectomycorrhizal fungi under different N fertilization levels (low-LN: 35 mg/plant, medium-MN: 60 mg/plant, high-HN: 120 mg/plant). For each inoculation treatment, different letters indicate significant differences (Contingency tables for percentage of mycorrhizal seedlings, Duncan’s test, p ≤ 0.05 for mycorrhizal colonization). |
| In the text | |
 |
Figure 2 Percentages of mycorrhizal seedlings (A) and mycorrhizal colonization index (B) on P. halepensis inoculated with three ectomycorrhizal fungi under different N fertilization sources (ammonium nitrate-NH4NO3, ammonium-(NH4)2SO4, nitrate-HNO3). For each inoculation treatment, different letters indicate significant differences (Contingency tables for percentage of mycorrhizal seedlings, Duncan’s test, p ≤ 0.05 for mycorrhizal colonization). |
| In the text | |


